ASTM F1980, also known as the Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices, provides multiple aging protocols to determine the effects of time on the physical properties of the package as well as the sterile integrity of the package. In the stability test, the Arrhenius equation is used, which states that a temperature increase of 10 °C will double the chemical reaction rate.
Stability Tests (Shelf Life Test) ASTM F1980
Other Tests
Test for Mammalian Chromosome Abnormalities
Chromosomal abnormalities (CA) refer to a numerical or structural change in the chromosome. CA test is used to detect various structural and numerical abnormalities by mutagens
Bacterial Reverse Mutation Test (AMES)
The Ames test determines whether a chemical is mutagenic or not.
Irritation and Skin Sensitivity Test
It is made to determine whether a medical device, material or chemical substance is irritating.
Gene Mutation Test (HPRT Test)
It is used to detect gene mutations induced by chemicals.
Hemolysis Test
Blood interaction tests are performed to determine the possible effects of medical devices that interact directly or indirectly with blood.
Cytotoxicity Test
Cytotoxicity is carried out with the aim of determining the toxic effects that can be caused by the contact of medical devices interacting with the body.
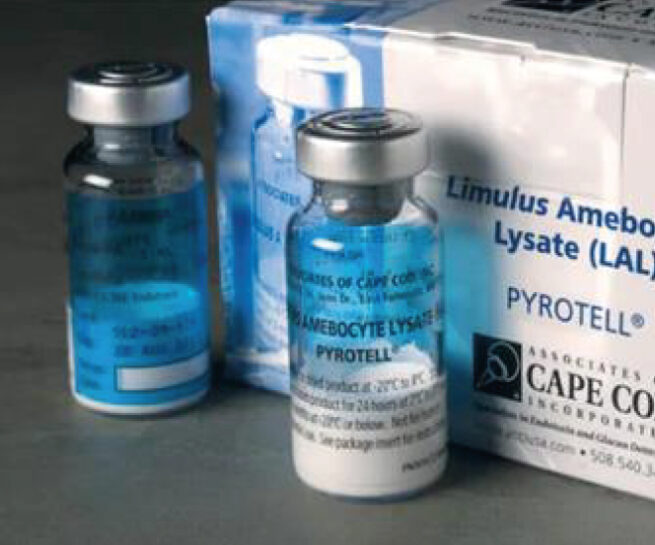
European Pharmacopoeie 11-2 Bacterial Endotoxin Test (2.6.14)
It is performed to detect toxic structures in the cell walls of gram-negative bacteria.
Stability Test
It is carried out to determine the time effects on the physical properties of medicinal products and their packages.
Bioburden Test
Bioburden is defined as the number of living microorganisms present in a given amount of material before medical equipment or devices are subjected to the sterilization procedure.
Sterility Tests
It is performed to detect the absence of live microbial cells in a sterilized product.